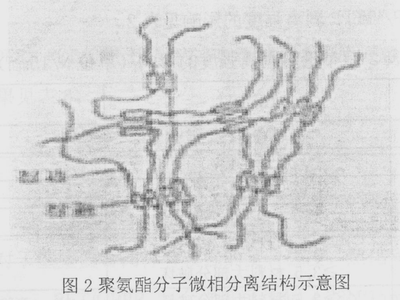
The two-component polyurethane adhesive is composed of a main agent comprising a hydroxyl group -OH and a curing agent comprising -NCO having an isocyanate group. When used, the main agent and the curing agent are mixed in a certain proportion, and after the molecules are extended by the chemical reaction, a high molecular weight polymer is formed. As an adhesive, the composition, molecular structure, and molecular weight distribution of the polyurethane have an important influence on the bonding. Polyurethane can be considered as a kind of block copolymer containing soft segment and hard segment [2]. The soft segment is composed of polyester, polyether, polyolefin and other oligomer segments, which is very compliant and shows random coiling state. Since the glass transition temperature is lower than room temperature, it is referred to as the rubber phase; the hard segment is composed of aromatic groups, substituted urea groups, carbamates, allophanates, biurets and the like, and is stretched at room temperature. Bar-shaped, it is not easy to change its conformational conformation, cohesive energy is large, and many microcells are associated with each other. As shown in Fig. 2, the glass transition temperature of these microcells is much higher than room temperature, and they are glass at room temperature. State, secondary crystal or microcrystal, called plastic phase. Due to the thermodynamic incompatibility of the two segments, microscopic phase separation occurs and phase or microphase regions form within the polymer matrix. The excellent properties of polyurethanes can be explained by the two-phase morphologies: the hard segments provide physical cross-linking of multiple functionalities, ie, the formation of hydrogen bonds and cross-linking, and play a reinforcing role: the soft segments are reinforced by the hard segments. Because the physical properties of aluminum, plastic film, etc. vary greatly, if the adhesive structure design is not reasonable, the stress generated during the curing process is concentrated; in addition, the two-component polyurethane adhesive is formed by the chemical reaction after the main agent and the curing agent are mixed. Molecular polymer (curing) bonding, this process takes a long time, usually requires 48h at room temperature. During this period, the residual monomers and small molecule oligomers in the adhesive will enter the interface between the VM layer and the CPP layer through the aluminum plating layer, and the VM layer will be reduced due to curing shrinkage of the adhesive and the stress caused by the aluminum and CPP wire expansion coefficients. The bond strength of the CPP layer.
2.2 Effect of material properties and aging conditions on peel strength
Three different thicknesses of the film are compounded and cured under different conditions. The peel strength is shown in Table 1 (unit: N/15mm).
Note: In Table 1, aging 1 refers to the storage time at around 50°C. CPP pull-off 2 means that most of the tested samples are broken. The measured peel strength data is 3.5 to 6.4 N/mm.
It can be seen from the table that the YH501SL/VM10 adhesive has a higher initial tack; composite films with different thicknesses have slightly different peeling strengths; the film thickness is larger and the peeling strength is also slightly larger. After 24 hours aging, the peel strength was very high. The peeling strength reached its maximum after aging for 48 hours, and the aging strength continued to decrease, and the aluminum plating layer was partially transferred. It is recommended not to exceed 48h during aging at 50°C.
We are manufacturer and supplier of Coffee Bean Canister,Tea Canister,Tea Container,Tea Storage Container,Stainless Steel Coffee Canister. And we located in Jiangmen, Guangdong, China. If any interested, please contact us for free.
Coffee Bean Canister,Tea Canister,Tea Container,Tea Storage Container,Stainless Steel Coffee Canister
Jiangmen Xinhui Zhancha Metal Products Co,. Ltd. , https://www.zcfoodmill.com